In fresh years, more and more medical investigations have subsidized an alarming speculation: Alzheimer’s illness is probably not simply a situation of an getting old mind, however the made from an infection.
While the precise mechanisms of this an infection are one thing researchers are nonetheless seeking to isolate, a lot of research counsel the fatal unfold of Alzheimer’s is going approach past what we used to assume.
One such find out about, printed in 2019, advised what may well be one of the crucial definitive leads but for a bacterial perpetrator at the back of Alzheimer’s, and it comes from a rather sudden quarter: gum illness.
Watch the video beneath for a abstract in their find out about:
In a paper led by senior author Jan Potempa, a microbiologist from the University of Louisville, researchers reported the discovery of Porphyromonas gingivalis – the pathogen behind chronic periodontitis (aka gum disease) – in the brains of deceased Alzheimer’s patients.
It wasn’t the first time the two factors have been linked, but the researchers went further.
In separate experiments with mice, oral infection with the pathogen led to brain colonization by the bacteria, together with increased production of amyloid beta (Aβ), the sticky proteins commonly associated with Alzheimer’s.
The research team, coordinated by pharma startup Cortexyme, which was co-founded by first author Stephen Dominy, wasn’t claiming to have discovered definitive evidence of Alzheimer’s causation.
But it was clear they thought we had a strong line of investigation here.
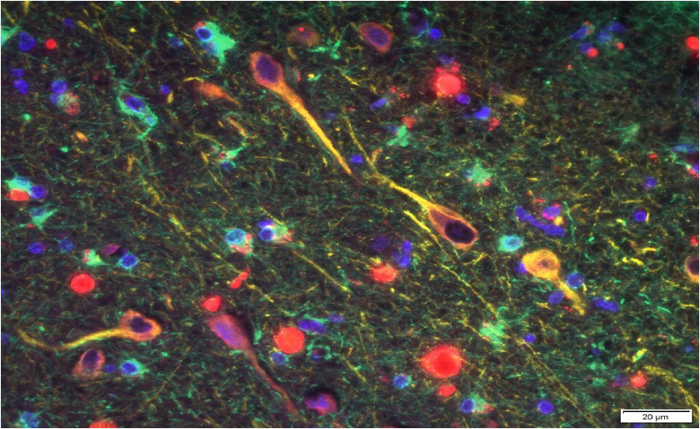 P. gingivalis’ gingipains (red) among neurons in the brain of a patient with Alzheimer’s. (Cortexyme)
P. gingivalis’ gingipains (red) among neurons in the brain of a patient with Alzheimer’s. (Cortexyme)
“Infectious brokers were implicated within the construction and development of Alzheimer’s illness sooner than, however the proof of causation hasn’t been convincing,” Dominy mentioned on the time.
“Now, for the primary time, we have now cast proof connecting the intracellular, Gram-negative pathogen, P. gingivalis, and Alzheimer’s pathogenesis.”
In addition, the team identified toxic enzymes called gingipains secreted by the bacteria in the brains of Alzheimer’s patients, which correlated with two separate markers of the disease: the tau protein, and a protein tag called ubiquitin.
But even more compellingly, the team identified these toxic gingipains in the brains of deceased people who were never diagnosed with Alzheimer’s.
That’s important, because while P. gingivalis and the disease have been linked before, it’s never been known – to put it simply – whether gum disease causes Alzheimers, or whether dementia leads to poor oral care.
The fact that low levels of gingipains were evident even in people who were never diagnosed with Alzheimer’s could be a smoking gun – suggesting they might have developed the condition if they had lived longer.
“Our id of gingipain antigens within the brains of people with AD and likewise with AD pathology however no analysis of dementia argues that mind an infection with P. gingivalis isn’t a results of deficient dental care following the onset of dementia or a end result of late-stage illness, however is an early tournament that may give an explanation for the pathology present in middle-aged people sooner than cognitive decline,” the authors explained in their paper.
Further, a compound formulated by the company called COR388, showed in experiments with mice that it could reduce bacterial load of an established P. gingivalis brain infection, while also reducing amyloid-beta production and neuroinflammation.
We’ll have to wait and see what future research will uncover about this link, but the research community is cautiously optimistic.
“Drugs focused on the micro organism’s poisonous proteins have thus far handiest proven get advantages in mice, but without a new dementia therapies in over 15 years it is important that we check as many approaches as conceivable to take on sicknesses like Alzheimer’s,” leader medical officer David Reynolds from Alzheimer’s Research commented in a commentary.
The findings had been reported in Science Advances.
An previous model of this tale used to be printed in January 2019.
 Global News Post Fastest Global News Portal
Global News Post Fastest Global News Portal














